
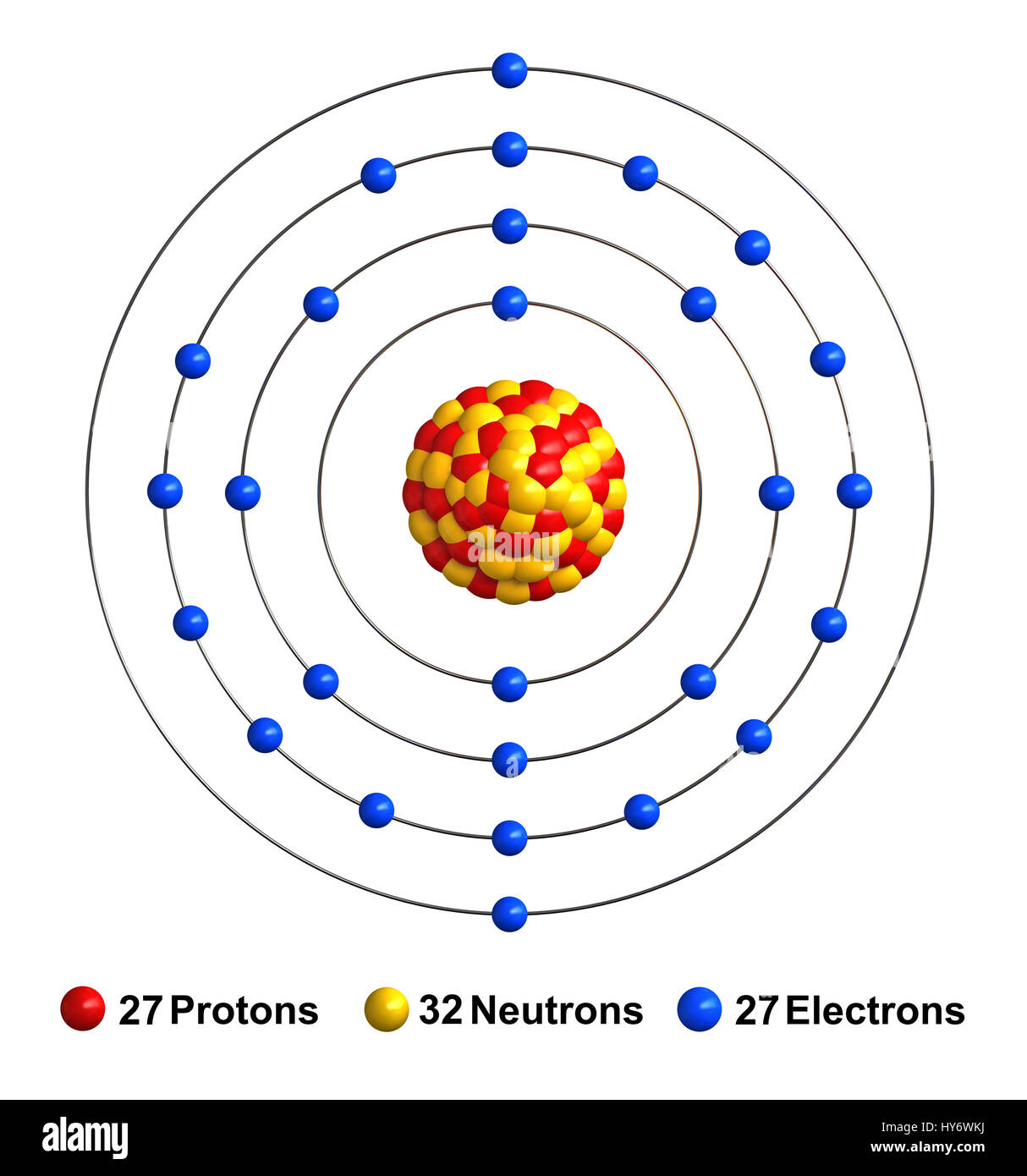
Now where would that one electron be? Well it would be in Has an atomic number of one which tells us it has one proton, and if it's neutral, that If we're talking aboutĪ neutral hydrogen atom, a neutral hydrogen atom, it And so first, let's just think about the electron configuration And to help us with that, we will look at a periodic table of elements. Now the goal of this video is to think about electron configurationsįor particular atoms. So if you're thinking about the subshell, the s subshell could fit two electrons, the p subshell can fit six electrons, the d subshell can fit 10 electrons, and the f subshell can fit 14 electrons, two per orbital.
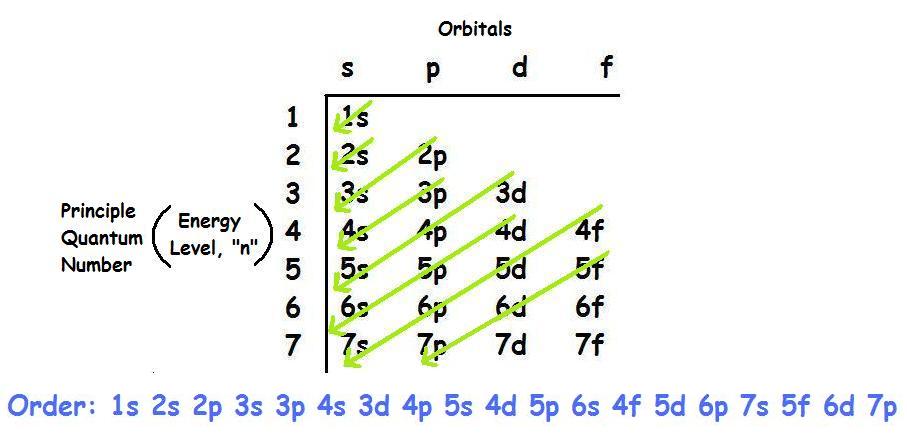
That has one, two, three, four, five different orbitals in it. So you have the s subshell, the p subshell that has threeĭifferent orbitals in it, you have the d subshell The various subshells which are found in the various shells. Us the types of orbitals which can be found in

And an orbital is a description of that, where is it more or In a previous video, we've introduced ourselves to the idea of an orbital, that electrons don't just orbit a nucleus the way that a planet might orbit a star, but really, in order to describe where an electron is atĪny given point in time, we're really thinking about probabilities, where it's more likely to be found and less likely to be found. This means that we have a total of 8 electrons in the 2nd energy level (2 from the s subshell, and 6 from the p subshell).Īs you go further, you will get practice with identifying and conceptualizing electron configuration. In every p subshell there are 3 p orbitals. In the 2nd energy level, we have a p subshell in addition to the s subshell. This specific type of orbital is called the s orbital, and we have 1 s orbital for every s subshell. In the 1st energy level, we have 1 subshell, which basically means we have 1 type of orbital. Each orbital then has 2 electrons, which are said to have different and opposite spins. Finally, within each subshell there are individual orbitals referencing a specific region of space around the atom's nucleus. We can organize these electrons into different subshells based upon the shape of the region they occupy. The pattern that we observe results in 3 classifications as follows (there are other considerations that you will learn about later as well).Ī Shell / Energy Level is a region or set of regions that have the same energy.Īlthough we cannot predict the exact location of the electrons at any time, we can map out the regions of space that they occupy. This pattern continues, but it starts to get more complicated with the inclusion of the d subshell and the transition metal elements beginning after calcium.Īs electrons are added to the space around the atom's nucleus they are arranged in a way as to minimize repulsions. The second electron shell is composed of an s and p subshell which has 4 orbitals altogether which is why period 2 element's valence shell holds 8 electrons. The first shell only has an s subshell which means it only has 1 orbital which is why period 1 elements valence shell only holds 2 electrons. An individual orbital can hold a maximum of 2 electrons.Įach electron shell has a certain amount of subshells (and therefore orbitals and therefore electrons it can hold). s subshells have 1 orbital, p has 3, d has 5, and f has 7. These subshells are themselves composed of orbitals which are the specific orbits of the electrons and each subshell has a certain number of orbitals. And we use the letters s, p, d, and f for subshells. The subshells which compose electron shells describe the general shape of electron's orbit around the nucleus. Electron shells are divided into subshells, which are further divided into orbitals. This is a decent starting point, but later you learn in chemistry that the placement of electrons is more complicated than this. And so if I'm understanding it correctly. Elements in period 2 (building on the previous 2 electrons) need 8 more electrons to fill the second electron shell which is their valence shell. So elements in period 1 (hydrogen and helium) need 2 electrons to fill their valence shell. So you're 2,8,8,2 rule looks like it shows the maximum amount of valence electrons elements can have in a period up to atomic number 20 (calcium).


 0 kommentar(er)
0 kommentar(er)
